TYPES OF BIOCHEMICAL TESTS USED
FOR BACTERIA:
- Tests for showing range of carbohydrates that can be attacked.
- Tests for specific breakdown products.
- Tests to show ability to utilize a particular substrate.
- Tests for metabolism of proteins and aminoacids.
- Test for enzymes
- Tests to distinguish between aerobic and anaerobic breakdown of sugars.
A. Biochemical tests to show the range of
carbohydrates
that can be attacked bacteria:
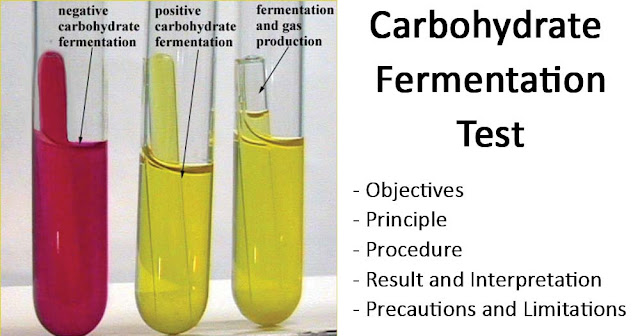
Sugar fermentation tests in which different sugars are used in l% concentrations:
(i) Monosaccharides - Glucose, fructose,
Mannose
(ii) Disaccharides - Sucrose, Lactose
(iii) Polysaccharides — Inulin, Glycogen.
(iv) Polyhydric alcohols — Glycerol,
Mannitol, Sorbitol, Dulcitol.
Indicators used are:
(i) Andrade’s indicator — Acid fuchsin in NaOH
(ii) Bromocresol purple
(iii) Bromothymol blue
(iv) Phenol red
Changes to be seen:
- If a particular sugar is attacked then either acid or acid & gas is produced.
- Acid can be detected by change in colour of medium. If Andrade’s indicator is used, colour becomes pink on acid production while gas is indicated by bubbling or pushing up of Durham’s tube in the medium.
B. Biochemical tests for specific breakdown
products:
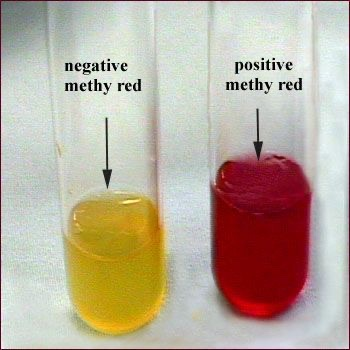
1. Methyl red test:
This test shows the production of sufficient
acid during carbohydrate fermentation and
maintenance of pH below 4.5
Organism is grown in glucose phosphate
peptone water for 48 h. at 37° C and methyl
red indicator’s 5 drops are added to culture.
Positive reaction — Bright red colour eg.
E.coli
Negative reaction — Yellow colour eg.
Klebsiella
2. Voges Proskauer test
This test detects the production of accetyl methyl carbinol from glucose and it gets oxidised to diacetyl on exposure to air & alkaline conditions, which gives pink colour with creatine.
Method : Take glucose phosphate peptone water culture of 48 h.Add l ml of 40% KOH & 3 ml of 5% α-napthol in absolute ethanol.Colour changes to pink.
Positive reaction - Pink (Klebsiella, Proteus)Negative reaction - Yellow (E. coli,
Salmonella)
C. Biochemical tests to show ability to utilize a specific substrate:
l. Citrate utilization test:
This test indicates the ability of organisms to utilise citrate as sole source of carbon. Koser’s citrate (liquid form) or Simmon’s citrate (solid lorm) medium can be used.
Method: Citrate is incorporated in the medium.
Inoculate the organism to be tested in the citrate
medium & incubate it for 16-24 hrs.
Koser’s medium
- Positive - Turbidity
- Negative - No turbidity
Simmon’s citrate medium
- Positive - Blue colour (Klebsiella) with visible growth.
- Negative - Green colour (E. coli) with no visible growth.
D. Biochemical tests for metabolism of proteins & aminoacids:
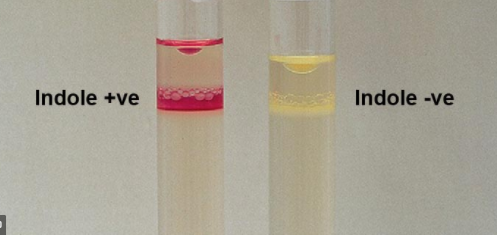
1. Indole production:
Certain bacteria decompose tryptophan amino acid to indole and this indole is detected by colorimetric reaction with P-dimethyl amino benzaldehyde. Reagent - Kovac’s reagent, which contains:
Amyl isoamyl alcohol.
P-dimethyl amino benzaldehyde.
HCI
Method: Inoculate peptone water and incubate for 48 hr at 37° C. Add 0.5 ml of Kovac’s reagent. A pink purple ring forms.
Positive reaction - Purple ring (E. coli,
Proteus)
Negative reaction - No ring (Klebsiella)
2. Gelatin liquefaction.
3. Hydrogen sulphide production test:
In this test ability of bacteria to decompose
sulphur containing aminoacids is detected.
Positive reaction - Black colored medium
Negative reaction - No change in medium
E. Biochemical tests for Enzymes
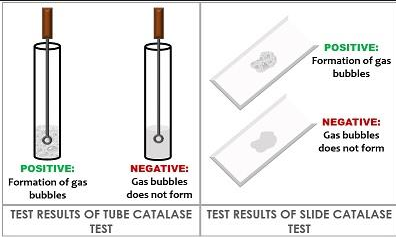
l. Catalase test:
This test demonstrates the presence of
catalase which catalyses the release of O2
from H202.
2H202 = 2H20 + O2——-—>O2 is detected
by bubbling in the tube.
Method: Take l ml of H2O2 solution. Put
small inoculum of bacterium by platinum
loop or glass rod. See for bubbling or
effervesence.
Positive - Bubbling/Effervescence
(Staphylococci, Vibrio)
Negative - No bubbling (Streptococci,
Pneumococci)
2. Oxidase test:
Principle:
This test detects the presence of oxidase in
bacteria which catalyzes the transport of
electrons between electron donors in bacteria
& redox dye (Tetra methyl paraphenylene
diamine dihydrochloride) TMPD.
The dye is reduced & gives a deep purple
colour.
Method: Soak the filter paper with solution of the reagent (TMPD) with a glass rod or wooden rod. Pick a part of bacterial colony and touch the filter paper.
Positive reaction - A purple colour develops
(Vibrio, Pseudomonas, Neisseria)
Negative reaction - No colour
3. Urease test:
The bacteria which contain urease,
decompose urea into ammonia.
Ammonia production is tested by means of a
suitable PH indicator.
Method : lnoculate urease medium which
contains urea with organisms & incubate it
for 24 h to 48 hr and observe for colour change.
Positive reaction - Pink colour (Klebsiella, Proteus)
Negative reaction - No colour
4. B-galactosidase test.
5. Nitrate reduction test.
6. Lecithinase test.
F. Biochemical test to distinguish between
aerobic and anaerobic breakdown of sugars:
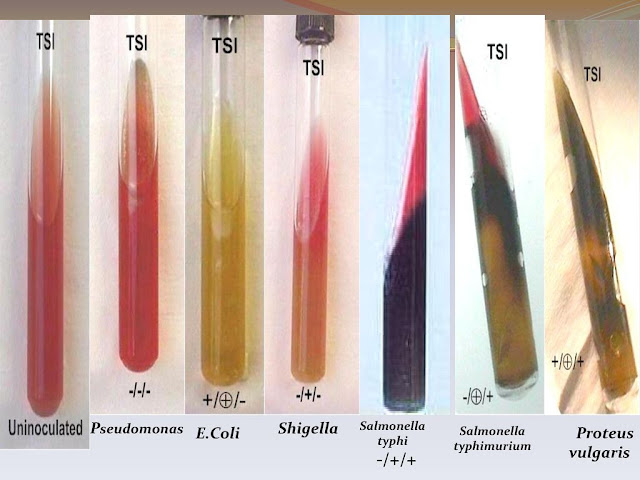
Triple sugar iron (TSI) agar test:
This agar contains:
- Yeast extract
- Peptone
- Glucose
- Lactose
- Sucrose
- Ferric citrate/Ferric ammonium citrate
- NaCl
- Sodium thiosulphate
- Agar
- Phenol red
- Distilled water
Observation:
1. Acid production due to breakdown of sugars
2. Gas production
3. H2 S production




No comments:
Post a Comment