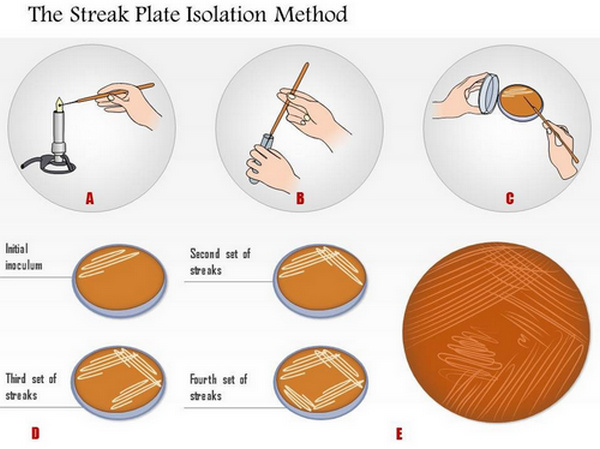
Collection and Transport of Blood and Body
Fluids for Culture
GENERAL
CONSIDERATIONS:
The proper collection of sample is the most
important step in the ultimate confirmation that a microorganism is responsible
for the infectious disease process.
Failure to isolate the causative organism in an
infectious process is not necessarily the fault of inadequate cultural methods;
it frequently results from faulty collecting or transport techniques.
So, following are the general considerations
recommended regarding the collection and transport of material for culture
- Whenever possible specimen should be collected
before antimicrobial agents have been administered.
- The material should be collected where the
suspected organisms is most likely to be found, with as little external
contamination is possible.
- Another factor that contributes in successful
isolation of organism is the stage of the disease at which the specimen is
collected for culture.
- Specimens should be of a sufficient quantity to
permit complete examination.
- The specimen should be placed in a sterile
leak-proof container to prevent hazard to the handling staff.
- Provision should be made for the prompt delivery of
specimen to the laboratory.
- One important thing is that clinicians should give
sufficient clinical information to guide the microbiologist in selection of
suitable media and appropriate technique.
- The clinicians must appreciate the limitations and
potentials of the bacteriology laboratory and realize that a negative result
does not necessarily invalidate the diagnosis.
- Laboratory personnel should reject specimens not
obtained in a proper manner.
- Each specimen must be clearly labelled with the
date and time of collection, patient’s name, number, ward or health centre.
- Each specimen must be accompanied by a request form
which includes the following:
- Patient’s name, age, number and ward or health centre
with name of consulting doctor.
- Type of specimen and date and time of its
collection.
- Clinical diagnosis with patient’s history.
- Patient`s immune status.
- Any antimicrobial treatment that may be started at home
or in the hospital.
- Specimens containing dangerous pathogens should be
labelled as HIGH RISK and immediately after collection they should be sealed
inside plastic bag or in a container with a tight — fitting lid.
- HIGH RISK specimens include:
- Sputum likely to contain M. tuberculosis.
- Faecal specimen that may contain V.cholera or S.
typhi.
- Specimens from patients with highly contagious
infections like HIV, Hepatitis, Viral haemorrhagic fever or plague.
BLOOD CULTURE
Blood culture is the most useful tool to detect
microorganism from cases
of pyrexia of unknown
origin.
DEFINITIONS:
- Bacteraemia: The presence of
bacteria in blood is called bacteraemia. It is usually pathological although
transitory asymptomatic bacteraemia can occur during the course of many
infections and following surgical procedures. Bacteraemia occurs in diseases
such as typhoid fever, brucellosis, leptospirosis and endocarditis.
- Septicaemia: The term
septicaemia refers to a severe and often fatal infection of the blood in which
bacteria multiply and release toxins into the blood stream. The commonest
portals of entry for bacteraemia/septicaemia are the genitourinary tract,
respiratory tract, abscesses, surgical wound infections, biliary tract and miscellaneous
sites.
COMMON PATHOGENS
CAUSING SEPTICEMIA
|
BACTERIA
|
GRAM POSITIVE: Staphylococcus aureus, Viridans streptococci,
Streptococcus pneumonia, Streptococcus pyogenes, Enterococcus faecalis,
Clostridium perfringens, Anaerobic streptococci.
|
|
|
GRAM NEGATIVE: Salmonella typhi, other Salmonella species, Brucella
species, Haemophilus influenza, Pseudomonas aeruginosa, Klebsiella species,
Escherichia coli, Proteus species, Bacteroides fragilis, Neisseria
meningitides, Yersinia pestis
|
|
OTHER BACTERIA
|
Mycobacterium tuberculosis, Leptospira species, Borrelia species,
Rickettsiae and Bartonella bacilliformis
|
|
FUNGI
|
Candida albicans and other yeasts and occasionally Histoplasma
capsulatum and other fungi that cause systemic mycoses
|
INDICATIONS FOR
BLOOD CULTURE
- Septicaemia, an often life —
threatening microbial invasion from an infected focus accompanied by increase
in temperature, increase in pulse rate or chills followed by fever.
- Bacteraemia, which accompanies
chronic infections such as disseminated gonococcal disease or severe infections
exemplified by meningitis, pneumonia, or deep seated abscesses.
- Intravascular infections such as
endocarditis, thrombosed blood vessels or those due to intravenous catheters.
- Bacteraemia of multisystem infections such as
enteric fever, leptospirosis, brucellosis.
- Bacteremia secondary to traumatic insults and instrumentation such as puncture wounds, urinary tract
catheterization, contaminated intravenous medication.
- Patients having fever of unknown origin (FUO).
TIMINGS FOR
COLLECTION OF BLOOD FOR CULTURE
- In conditions like undrained abscesses,
instrumentation of contaminated mucosal surfaces, manipulation of infected
tissues, bacteria are transiently present in the blood stream.
- During early stages of typhoid fever, brucellosis,
bacteria are continuously present in the blood stream.
- While in bacterial endocarditis, septic shock,
organisms are released into the blood stream at fairly constant rate making
timing of cultures unimportant.
- So blood should be collected at the time of the
patient’s temperature beginning to rise. (Except bacterial endocarditis where
blood can be collected at any time).
MEDIA FOR BLOOD
CULTURE
- Blood culture media should contain a nutrient broth
and an anticoagulant.
- (i) Tryptic soya broth.
- (ii) Brain heart infusion broth.
- (iii) Thioghycollate broth.
COLLECTION OF BLOOD
- Blood is collected by proper phlebotomy. Skin
should be cleaned properly before collecting blood, this is necessary to reduce
risk of introducing contaminants into blood culture media.
- It is less desirable
to draw blood through a vascular shunt or catheter since these prosthetic
devices are difficult to decontaminate completely.
- It is also recommended to draw blood below an
existing intravenous line if possible, since, blood above the line will be
diluted with the fluid being infused.
- If the blood is not being inoculated directly into
broth media, it must be transported with an anticoagulant sodium polyanethol
sulfonate (SPS, Liquoid).
- SPS is also anticomplementary, antiphagocytic and
interferes with the activity of some antimicrobial agents, notably aminoglycosides.
Heparin, EDTA and Citrate have been found to be inhibitory to a number of
organisms.
Method:
Total aseptic precautions, just like minor surgical
preparations are required.
(i) Using a pressure cuff, locate a suitable vein
in the arm.
(ii) Cleanse thoroughly the skin over the vein
using tincture of iodine followed by ethanol/ether.
(iii) Remove the protective cap from the top of the
culture bottle and cleanse the top of each bottle using an ethanol/ether swab.
(iv) Using a sterile syringe and size 2l/suitable
gauge needle withdraw 10 ml of blood.
(v) With care, remove the needle from the syringe
and dispense the blood into culture bottle in the vicinity of a flame.
(vi) Using an ethanol/ether swab, wipe the top of
culture bottle and replace the cap. Gently mix the blood with the broth
(Blood/broth volume should be atleast 1:10).
N.B.: The blood must not be allowed to clot in the
culture media because any bacteria will become trapped in the clot.
(vii) Label the bottle with name, registration
number of patient, ward, unit, date and time of collection.
(viii) As early as possible, incubate the
inoculated media at 35 ° — 37° C.
INCUBATION
Incubate at 35°C — 37°C for upto 7 days with
regular examining and subculturing as per guidelines.
SIGNS OF BACTERIAL
GROWTH
Examine daily for upto 7 days.
(i) Turbidity above the red cell layer.
(ii) Haemolysis of the red blood cells.
(iii) Gas bubbles.
(iv)Appearance of small colonies in the broth on
the surface of sedimented red cell layer or along the wall of bottle.
A sterile culture usually remains clear. A slight
turbidity may develop after several days of incubation.
lf there are signs of bacterial growth, subculture
the broth and examine a Gram stained smear for bacteria and then do standard
follow up for identification of organism.
BACTEC
- The BACTEC blood culture system is a fully
automated microbiology growth and detection system designed to detect microbial
growth from blood specimens.
- The BACTEC™ FX and the BACTEC™ 9000 family of
continuous monitoring blood culturing instruments offering performance, safety,
reliability, ease of use, media quality and service.
- The BACTEC 9000 series of blood culture instruments are
designed for the rapid detection of microorganisms in clinical specimens. The
sample to be tested is inoculated into the vial which is entered into the BACTEC
instrument for incubation and periodic reading.
- Each vial contains a sensor which responds to the concentration of CO2
produced by the metabolism of microorganisms or the consumption of oxygen
needed for the growth of microorganisms.
- The sensor is monitored by the
instrument every ten minutes for an increase in its fluorescence, which is
proportional to the increasing amount of CO2 or the decreasing amount of O2
present in the vial.
- A positive reading indicates the presumptive presence of
viable microorganisms in the vial.
COLLECTION AND TRANSPORT OF VARIOUS BODY FLUIDS
COLLECTION AND
TRANSPORT OF EFFUSIONS
- Synovial, Pleural, Pericardial, Ascitic and
Hydrocele fluids
- An effusion is fluid which collects in a body cavity.
Fluids which collected due to an inflammatory process is referred as an exudate
and that which forms due to a noninflammatory process is referred as a
transudate.
- Collection and transport of effusions:
- After aspiration, aseptically dispense the
fluid as follows:
- 2 — 3 ml into dry, sterile, screw — cap tube or
bottle.
- 9 ml into a screw — cap tube or bottle which
contains l ml of sodium citrate solution. Mix the fluid with the anti -
coagulant.
- An anticoagulant is required to prevent clotting,
especially exudates. The citrated samples can be used for the cell count,
protein estimation, microscopy and culture. A sample without citrate is useful
to see whether clotting occurs.
- Label it properly and deliver the specimen
with a request form to the laboratory as early as possible.
- If there is delay in transport of specimen,
then dispense 5 ml of fluid into a bottle of sterile thioglycollate broth and
mix.
COLLECTION AND
TRANSPORT OF CSF
- Cerebrospinal fluid must be collected by an
experienced Medical Officer or health worker. It must be collected aseptically
to prevent organisms being introduced into the central nervous system. The
fluid is usually collected from the arachnoid space.
- A sterile wide bore needle is inserted between the
fourth and fifth lumbar vertebrae and the CSF is allowed to drip into a dry
sterile container. A ventricular puncture is sometimes performed to collect CSF
from infants.
- CSF should be examined as early as possible. A
delay in examining CSF reduces the chances of isolating a pathogen. It will
also result in a lower cell count due to WBCs being lysed, and to a falsely low
glucose value due to glycolysis.
- When trypanosomes are present, they will be
difficult to find because they are rapidly lyzed once the CSF has been
withdrawn.
COLLECTION OF CSF
1. Take two sterile, dry, screw-capped containers and label one No.1 (first
sample for culture purpose) and No.2 (second sample for other investigations).
2. Collect about 1 ml of CSF in container No.1 and about 2-3 ml in
container No.2.
3. Immediately deliver the samples with a request form to the laboratory.
COMMON PATHOGENS IN
CSF
|
BACTERIA
|
GRAM POSITIVE: Streptococcus pneumoniae, Streptococcus agalactiae,
Listeria monocytogenes.
|
|
|
GRAM NEGATIVE: Neisseria meningitides, Haemophilus influenza type b,
Escherichia coli, Pseudomonas aeruginosa, Proteus species, Salmonella
species,Flavobacterium meningosepticum
|
|
OTHER BACTERIA
|
Mycobacterium tuberculosis, Treponema pallidum
|
|
FUNGI
|
Cryptococcus neoformans (mainly in AIDS patients) and less commonly
Aspergillus species
|
|
VIRUSES
|
Coxsackieviruses, echovirus, and arboviruses, herpes simplex 2 virus,
varicella zoster virus and Lymphocytic choreomeningitis virus (LCM). Rarely
polio viruses.
|
|
PARASITES
|
Trypanosoma species and Naegleria fowleri. Also Toxoplasma gondii (in
AIDS patients)
|