STAINING OF BACTERIA
INTRODUCTION
Bacteria cannot be seen under the light microscope without staining due to the lack of contrast, until special methods of illumination are used.
IMPORTANCE OF STAINING
- The microscopic examination of stained smear enables the morphology, relative sizing & arrangement of microorganism to be seen clearly. It also assists in the detection of cells, esp. pus cells.
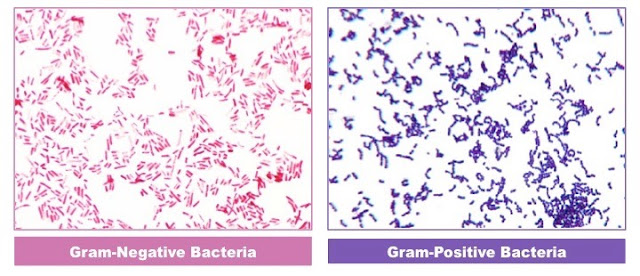
- Bacteria can also be differentiated by their staining reactions, e.g. gram positive from gram negative organisms.
DEFINITIONS
Supravital Stain
Stains which are toxic and kill the cell on staining.
Vital Stain
Nontoxic stain during which cells retain their viability.
TYPES OF STAIN
Simple Stains
Definition: Watery solution of a single basic dye is used to stain the smear.
Example: Methylene blue or basic fuchsine
Use: They just demonstrate the presence of bacteria in the smear
Disadvantage: Since only one stain is used, it is not possible to say whether the organism is gram positive or gram negative.
Negative Stains
Definition: Bacteria are mixed with dyes. The background gets stained leaving the bacteria contrastingly colourless.
Example: Indian ink, Nigrosin
Use: For demonstration of bacterial capsule.
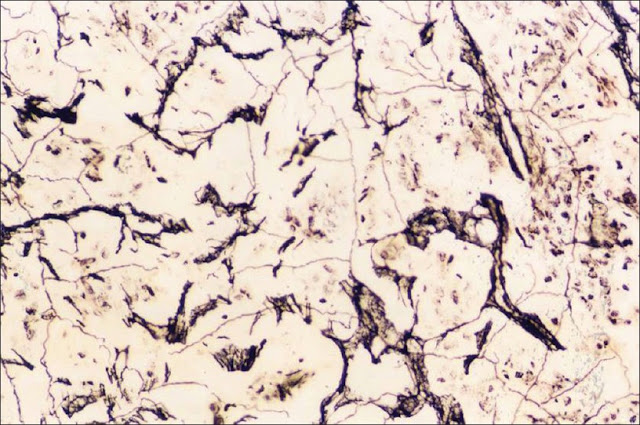
Silver Impregnation Method
Definition: Bacterial cells and appendages that are too thin and delicate cannot be seen under ordinary microscope. These delicate structures are thickened by impregnation of silver on the surface to make them visible under light microscope.
Use: To demonstrate spirochaetes and bacterial flagella.
Differential Stains
Definition: They impart different colours to different bacteria or their structures.
Example: Gram stain and ZN stain.
Use: Gram stain differentiates bacteria into gram positive and gram negative. Acid fast stain (ZN stain) differentiates bacteria into acid fast and non acid fast.
Special Stains
Definition: They are devised for some special purpose, to identify some particular bacteria or its component.
Example: Albert’s stain, Neisser’s stain and Ponder’s stain for Corynebacterium diphtheriae.
Use: They demonstrate metachromatic granules of corynebacterium diphtheriae.
LABELLING OF SLIDES
Every slide should be labelled clearly with number or letters by diamond or grease pencil. Stick—on paper labels should not be used as they are liable to be soiled or washed off in staining procedures.
PREPARATION OF SMEAR FOR STAINING
A bacterial smear is a dried preparation of bacterial cells on a glass slide. In a bacterial smear that has been properly processed-
(1)The bacteria are evenly spread out on the slide in such a concentration that they are adequately separated from one another, (2) The bacteria are not washed off the slide during staining, and
(3) Bacterial form is not distorted.
Smears should be spread evenly covering an area of about 15-20mm diameter on a slide.
1. From purulent specimen (pus, turbid urine etc.).
Take a loopful of specimen with the sterile inoculating wire and spread thinly on the slide.
2. From non purulent specimen.
Centrifuge the specimen & make a smear from a drop of the well mixed deposit.
3. From Sputum.
Use a piece of clean stick to transfer and spread purulent & caseous material on a slide. Soak the stick in phenol before discarding it.
4. From swab.
Roll the swab on the slide.
5. From culture plate.
Put a drop of saline on a clean slide. With the help of sterile inoculating wire, a individual colony is picked up & mixed with saline drop to prepare a thin emulsion.
In making a smear from culture, bacteria from either a broth culture or an agar slant or plate may be used. If a slant or plate is used, a small amount of bacterial growth is transferred to a drop of water on a glass slide and mixed. The mixture is then spread out evenly over a large area on the slide.
FIXATION OF SMEAR
PURPOSE
The purpose of fixation is to preserve microorganisms, and to prevent smears being washed from slide during staining. Smears are fixed by heat or alcohol.
NOTE: Microorganisms are not always killed by heat fixation. So please handle them carefully, considering them to be infective. Discard the slides after use in 5% phenol.
HEAT FIXATION
1. Allow the smear to air-dry completely.
2. Rapidly pass the slide; smear uppermost, three times through the blue flame of the spirit lamp or pilot Flame of Bunsen burner.
3. Allow the smear to cool before staining.
Disadvantage of heat fixation:
- Integrity and morphology of the organism may be disturbed & staining reactions altered especially where excessive heat is used.
- It also damages leucocytes. So it is unsuitable for fixing smear which contains intracellular organism such as N. gonorrhoea & N. meningitides.
ALCOHOL FIXATION
1. Allow the smear to air dry completely
2. Add one or two drops of absolute methanol or ethanol
3. Leave the alcohol on smear for a minimum 2 min. or until the alcohol dries on the smear.
Advantages of alcohol fixation:
- Less damaging to micro organism than heat.
- Pus cells are well preserved. So alcohol fixation is recommended for fixing the smear when looking for gram negative intracellular diplococci.
- It is more bactericidal than heat so it is preferable to fix the smear prepared from sputum.
- (M. Tuberculosis)
OTHER CHEMICAL FIXATIVES
1. Potassium permanganate. (Anthrax bacilli)
2. Formaldehyde vapour (Mycobacterium species)

No comments:
Post a Comment