DISCOVERY
The Ziehl-Neelsen acid-fast staining procedure was developed by Franz Ziehl, a German bacteriologist, and Friedrich Neelsen, a German pathologist, in 1881.
Pink tubercle bacilli
CLASSIFICATION
It is another differential stain used mainly to detect mycobacteria that cause tuberculosis, leprosy & opportunistic infections. Modified ZN staining is utilized to identify actinomyces, nocardia and oocysts of some intestinal protozoa.
PRINCIPLE OF ACID FAST STAIN
Some bacteria after being stained by carbol fuchsin resist decolorization by dilute mineral acids and appear red. These are known as acid fast bacteria. Whereas those decolourised after primary staining, take the counter stain & appear blue are known as non-acid fast bacteria.
MECHANISM OF ACID FAST STAIN
Acid fast organisms have high molecular weight fatty acids called Mycolic acids which are attached to branched chain polysaccharide called arabinogalactan: Resistance to decolorization by acid-alcohol is associated with this mycolic acid-arabionogalactan moiety that constitutes the capsule surrounding the peptidoglycan layer.
PROCEDURE OF ACID FAST STAIN
1. Alcohol fix smear is placed on staining rack.
2. Cover the smear with the filtered carbol fuchsin stain.
3. Heat the stain-covered slide with help of burner till vapour comes out. Allow the heated stain to remain on the slide for 5 minutes. Note: While giving heat two things should be kept in mind. One that the stain should not dry. If it starts drying then add carbol fuchsin immediately. Second that it should not be boiled. Gently heat only till vapour just start coming out, then stop. Heating is done 2-3 times during the 5 minutes.
4. Wash off the stain with clear water.
5. Decolorize with acid till no more pink stain appears in washing.
Note: Acid alcohol is flammable, therefore use with care well away from an open flame.
6. Wash with clean water.
7. Counter stain with methylene blue solution for 1-2 minutes.
8. Wash off the stain with clean water.
9. Wipe the back of the slide clean, and place in a draining rack for a smear to air-dry.
10. Examine the smear microscopically with oil immersion objective.
Interpretation of Acid Fast Stain:
Acid Fast bacilli (AFB) - Red
Straight or slightly curved rods, occurring singly or in small groups.
Non Acid Fast bacteria - Blue
Cells - Blue
Background material - Pale blue
MODIFICATIONS OF ACID FAST STAIN WITH EXAMPLES
Any modification in the acid fast staining where different percentages of H2SO4 is used (other than 20% H2SO4 ) is known as modified acid fast staining.
Revised National Tuberculosis Control Programme (RNTCP) advocates use of 25% H2SO4 as a decolouriser for M.tuberculosis.
1. Leprosy bacilli are also acid fast, but usually to a less degree than tubercle bacillus. So here instead of 20% sulphuric acid, a 5% sulphuric acid is used for decolorization.
2. Sections of tissues containing ‘clubs‘ caused by Actinomyces, Mycobacterium & Nocardia can be treated with- 1% H2SO4 to demonstrate the acid-fastness of the clubs.
3. Cultures of some Nocardia species are acid fast when decolorized with 0.5% H2SO4.
4. Most Spores are acid fast with 1% H2SO4
ALTERNATIVE REAGENTS
Decolourizers: Besides 20% H2SO4, other decolouriser that can be used is 3% HCl in 95% alcohol.
Counter stains: Besides methylene blue, malachite green and picric acid are alternative counter stains for ZN stain.
COLD ZN STAIN
Carbol fuschin is not heated in this methods but concentration of phenol is increased to 4%. It is just allowed to remain in the slide for 5 mins. And then washed in the tap water. It is used for visualizing cryptosporidium oocysts in the faecal smear. It is also known as Kinyoun’s method.
Where large number of smears are to be examined, smears stained by fluorescent stains such as auramine phenol or auramine rhodamine are examined under ultraviolet light. The bacilli appear as bright rods against dark background. This method is more sensitive but requires a fluorescent microscope.
OTHER STAINS:
ALBERT'S STAINING
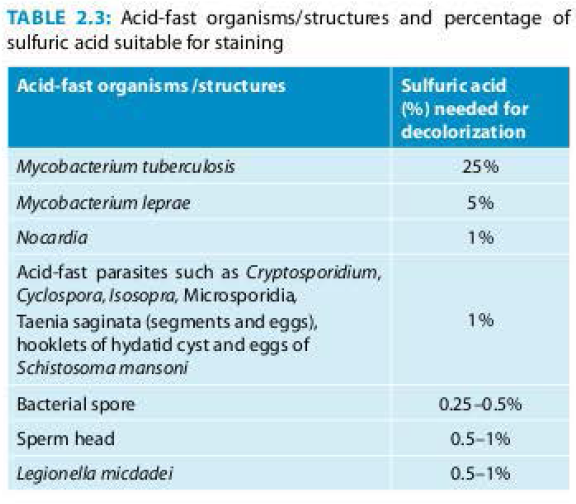
Albert's staining is most common staining technique used for examining the specimens suspected to have Corynebacterium diphtheriae; which are gram positive rods of varying length and often containing volutin (polyphosphate) granules which appear purple black against the light green cytoplasm of the bacillus.
ROMANOWSKY STAINS
Water based - Giemsa stain, Field’s stain, JSB (Jaswant, Singh & Bhattacharji)
Alcohol based - Leishman’s
GIEMSA STAIN
Giemsa stain
Thick and thin smears are prepared and stained by Giemsa stain and observed under oil immersion lens.
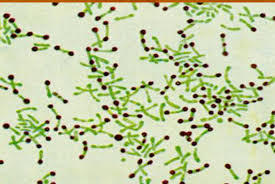
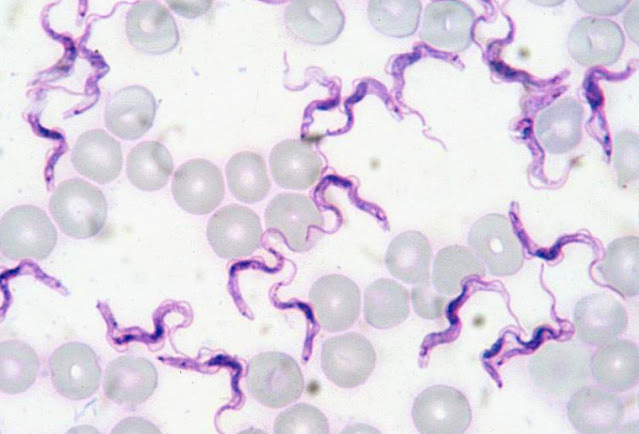
Use: For malaria, leishmania etc.
NEGATIVE (INDIA INK) STAINING
Cryptococci in negative staining
Negative staining is sometimes a very useful technique for demonstrating the capsulated organisms like Meningococcus/Pneumococcus/Cryptococcus, the causative agents of meningitis. Principle – The organism does not absorb the stain. It is seen as colourless object against coloured background. The preparation is examined under low power and high power lens.
FIELD’S STAIN
Field's stain
Field stain is a histological method for staining of blood smears. It is a rapid method used for staining thin and thick blood films in order to discover malarial parasites. Field's stain is a version of a Romanowsky stain, used for rapid processing of the specimens. It uses methylene blue and Azure 1 dissolved in phosphate buffer solution, and Eosin Y in buffer solution.



No comments:
Post a Comment