Preface:
Intestinal involvement is
the most common presentation with parasitic infections. Many parasites can
cause extra-intestinal diseases. Few intestinal parasites may also lead
extra-intestinal complications. Microscopic evidence in the form of peripheral
blood smear examination is the most common modalities for diagnosis of such
extra-intestinal infections with parasites. However other modalities like
immunological tests and molecular techniques may help in diagnosis of
extra-intestinal parasitic diseases.
Learning objectives:
·
List of the extra intestinal infections
in human caused by parasites.
·
List of the parasites those can be
found in peripheral blood smears.
·
Preparation, examination and
interpretation of an ideal peripheral blood smear.
·
Brief discussions on other methods
available to diagnose those infections.
List of the extra intestinal infections in human
caused by parasites.
1.
Malaria
2.
Filaria
3.
Kala-azar
4.
Toxoplasmosis
5.
Cysticercosis
6.
Hepatic amoebiasis
7.
Hydatid cyst
8.
Free living amoebic infections
9.
Trichomoniasis
List of the parasites those can be found in
peripheral blood smears.
1.
Plasmodium (causative agent of Malaria)
2.
Babesia (zoonotic and opportunistic infection)
3.
Trypanosoma (Chaga’s disease and Sleeping sickness)
4.
Leishmania Kala-azar/ Leishmaniasis)
5.
Wuchereriabancrofti (Lymphatic and occult filariasis)
6.
Brugiamalayi (Brugianfilariasis)
7.
Loa loa (African eye worm disease)
8.
Mansonella
Preparation,
examination and interpretation of an ideal peripheral blood smear.
Collection of blood:
Capillary
blood can be collected by finger pricking. Venous blood may be collected in
tube containing anticoagulant like EDTA which should be examined as soon as
possible.
Blood should be collected as soon as possible
in suspected case of malaria especially before administrating anti malarial
drugs.
Nocturnal periodicity: Microfilariae are usually present in
greatest numbers in the peripheral blood during night hours, which correspond
to the peak biting times of their insect vectors hence blood should be
collected during night hours.
Methods of examination of blood :
o
Direct wet mount
examination.
o
Examination of
blood smear after permanent staining.
o
Examination of
buffy coat region. (quantitative buffy coat)
o
Concentration of
blood.
Direct wet mount examination.
Place a drop of blood, collected by finger
prick, on a glass slide and cover it with coverslip. Examine under low power
objective lens of microscope.
·
For detection of
microfilariae and trypanosomes by their motility.
·
Counting of
microfilariae by examining on a Neubauer chamber.
Permanent staining.
Thick and thin, two types of smears can be
prepared with their own merits and demerits for the purpose of permanent
staining. Such stained smears are to be examined under oil immersion field.
Methods for preparation of peripheral blood
smear:
Thick smear:
-
A drop of blood in middle of the slide.
-
Using a pipette or stick, the blood is evenly distributed over the area of
about 15 X 15 mm on the slide.
-
It should be possible to just see (but not read) the news print on the paper
through the smear.
-
Label the slide legibly.
-
Smear is allowed to dry. It needs not to be fixed.
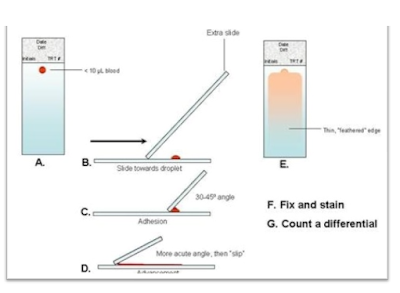
Thin smear:
-
A small drop of blood is placed near one end of slide.
-
A dry grease free glass slide (or a smooth edged slide spreader) is kept at an
angle over the blood drop, which in turn will spread along the entire edge of
spreader that is in contact with blood on slide.
-
The spreader is now rapidly moved over the surface of slide to make a thin
blood film.
-
A good thin smear should have a smooth tail and be free of vertical lines and
holes.
-
It should be possible to read the newsprint on a paper through the smear.
-
The smear is fixed with absolute methanol or absolute ethanol for 1 minute and
then allowed to dry.
|
Thick smear |
Thin smear |
|
RBCs are lysed. |
RBCs are fixed, mostly
in a single layer |
|
Larger volume
of blood can be examined. 0.25 µl/ 100 fields. |
Less volume can be
examined. 0.005 µl/ 100 fields. |
|
Blood elements
are more concentrated, hence species identification of Plasmodium not done. |
Good for species
identification and differentiation of Plasmodium. |
|
Good screening
test. |
More time consuming ,
not appropriate for screening. |
Note:
Thick and thin smear may be prepared on the same slide with due care.
Stains:
·
Romanowsky’s
stains include Giemsa, Leishman’s, Wright’s, Fielde’s and Jaswantsingh and
Bhattacharya’s stains.
·
These stains
contain combination of methylene blue (basic) which stains the nucleus and
eosin (acidic) which stains the cytoplasm.
·
They also contain
oxidation product of methylene blue called azures; which provide further
contrast.
Giemsa stain-
·
The aqueous
working solution prepared from Giemsa stain powder in various dilutions i.e.
1:10, 1:20, 1:30 etc. is prepared daily according to Standard Operative
Procedure prepared by the laboratory.
·
Fixed thin smear
or dried unfixed thick smear is immersed in the working solution in copulin’s
jar for 10, 20 or 30 mins depending upon the prepared dilution of giemsa
working solution.
·
Slide is washed
with phosphate buffer or tap water and examined.
Fielde’s stain-
Fill up two couplin jars with Field
stain A and Field stain B separately.
- Dip the smear in Field stain B for
5-6 seconds and wash in running water.
- Dip the smear in Field stain A for
20-30 seconds and wash in running water.
- Wipe the back of the slide clean.
- Air dry the smear and examine under
microscope.
Leishman’s stain-
-Stain contains Leishman’s powder in
absolute ethalnol. It is kept under sunlight or in incubator for 1 hour for 3 days
in a glass stoppered brown bottle for maturation.
-The smear is poured with about 10
drops of stain for 2 minutes followed by adding up of double the volume of
distilled water and mixed gently by rocking the slide.
-After 15 minutes, the slide is washed
with buffered distilled water and air dried for examination under the
microscope.
Jaswant-Singh-Bhattacharya stain (JSB stain)-
-Standard method used by the
laboratories under the National vector borne disease control program in India.
(NVBDCP)
-Stain has two solutions, solution 1
and solution 2.
-Fixed and air-dried smear is immersed
in solution 1 for 30 seconds and then washed with water.
-Slide is immersed in solution 2 for 1
second and washed with water.
-Slide is immersed again in solution 1
for 30 seconds. Slide is washed again as above till the smear gives pink
background, then air dried to be examined under microscope.
Quantitative buffy coat (QBC):
It is an advanced microscopic technique for
malaria diagnosis consisting of mainly three steps:
1. Concentration of blood by centrifugarion
2. Staining with acridine orange stain
3. Examination under ultraviolet (UV) light
source.
•
Commercially available quantitative buffy coat
capillary tube is precoated internally with acridine orange stain.
•
60 µl of peripheral blood is collected in the tube.
•
The tube is centrifuged at 12000 rpm for 5 minutes
with a float in it.
•
The components of blood are separated according to
their densities, forming discrete bands.
•
The buffy coat region i.e. at the RBC/WBC interface is
examined under UV light.
The advantages are: faster, more sensitive and quantification is
possible.
Disadvantages are its poor cost effectiveness, less specificity and
difficult and cumbersome procedure.
Brief discussions on
other methods available to diagnose Malaria
Detection of
malarial antigens or enzymes in serum by rapid tests
Several malaria
antigens and enzymes can be detected by Rapid Diagnostic Test (RDTs) like:
Parasite lactate
dehydrogenase (pLDH)- It is produced by trophozoites and gametocytes. Currently available
kits can differentialte between pan malarial pLDH and pLDH specific to
P.falciparum.
Plasmodium
falciparum specific histidine rich protein-2 (Pf-HRP-2)- Produced by
trophozoites and young gametocytes of P falciparum.
Parasite aldolase- Produced by all
Plasmodium species.
Most of the kits are
designed to detect a combination of two antigens, one is P.falciparum specific
and the other is pan malarial antigen.
Advantages
of RDTs
1.
Simple and rapid.
2.
More than 90% sensitivity if >100 parasites/µl
3.
pLDH can be used to monitor response of treatment.
Disadvantages
of RDTs
1.
Gametocytes can not be detected.
2.
False positive bands appear in Rheumatoid arthritis factor positive
cases.
3.
Ineffective in less than 40 parasites/µl for HRP2 detection and in less
than 100 parasites/µl for pLDHdetection.
Methods to
diagnose filaria.
Detection of microfilariae by microscopic
examination.
i. Demonstration of microfilariae in
smear prepared from peripheral blood collected at correct time (10 p.m to 4
a.m, peak 2 a.m.)
ii. Detection of microfilariae in
blood film after concentration by centrifugation technique
iii. Detection of microfilariae in
urine, hydrocele fluid or other body fluids.
2. Detection of Adult worm by lymph node
biopsy.
3. Detection of filarial antigens in serum
4. Detection of antibodies
5. Molecular method.
Speciemen
collection & methods to diagnose other parasitic infections:
Urogenital
specimens
These include vaginal and urethral discharges;
and prostatic secretions. These specimens are frequently examined for
identification and detection of Trichomonasvaginalis.
Sputum
Sputum is collected and examined commonly for
the demonstration of eggs of Paragonimuswestermaniin the pulmonary
paragoimiasis and less frequently, for trophozoites of E. histolyticain
the cases of amoebic pulmonary abscess.
Aspirates
The examination of aspirated materials is
recommended in the diagnosis of certain parasitic infections. Microscopic
examination of the wet mount of pus from the liver or lung amoebic abscesses is
frequently carried out for the demonstration and identification of amoebic
trophozoites.
In cystic echinococcosis, the aspiration of
hydatid fluid from the liver or lung hydatid cysts, after their surgical
removal, is carried out for the parasitic diagnosis of the condition. The
parasitic diagnosis is made by microscopic demonstration hydatid sand
(scolices) or hooklets in the wet-mount preparation of the hydatid fluid.
Aspirated materials collected from bone
marrow, spleen, liver, lymph nodes or CSF are usually examined for the presence
of trypanosomes or leishmanial parasites.
Cerebrospinal
fluid
Direct wet-mount examination of the CSF is
useful for the demonstration of motile free-living amoebae, Naegleria
and Acanthamoeba, the causative agents of primary amoebic
meningoencephalitis.
Biopsy
specimens
Pneumocystis jeroveci can be demonstrated in impression smears of
lung specimen obtained by open, trans-bronchial or brush biopsy and of
tracheobronchial aspirates and bronchoalveolar lavage. Cryptosporidium
sp. in addition to their presence in stool specimens can also be demonstrated
in the Giemsa-stained smear of jejunal biopsy specimen by routine histology or
culture.
Examinations of skin biopsy specimens are
useful for the diagnosis of cutaneous amoebiasis or cutaneous leishmaniasis.
The examination of skin-snips confirms the diagnosis of onchocerciasis.
Diagnosis of trichinellosis is confirmed by
the examination of muscle biopsy.
Diagnosis of schistosomiasis is also sometimes
confirmed by the demonstration of schistosome eggs in the rectal bladder
mucosal biopsy specimens.
Do microscopic examination of stained
peripheral blood smear for detection of parasite & draw labeled diagram of
significant microscopic findings


No comments:
Post a Comment