Preface:
Diagnosis
of fungal infections is often made by clinical appearance, but laboratory
investigations can help when
- the diagnosis is uncertain or
- the infection is chronic, severe
or invasive,
- Considering systemic anti fungal
therapy.
Learning
objectives:
-
Classification of fungal infections
& their aetiologies
-
Specimen collection in different
type of fungal infections
-
Microscopic & staining methods
for detection of fungi in specimen
o KOH
preparation
o Negative
stain
o Calcofluor
white stain
o Histopatholgy
of tissue
o PAS
/ GMS stain
o Gram
stain
-
Culture methods & media used
& growth pattern of different fungi over them.
-
Other ( immunological &
molecular methods) for diagnosis
The
comprehensive diagnostic approach to fungal infections can be divided under two
broad headings:
A.
Clinical diagnosis
B. Laboratory diagnosis
Clinical diagnosis
The
clinical criteria may give presumptive diagnosis of fungal infections.
Clinical classification of fungal infections (mycoses)
& their causative agents
|
Mycoses |
Definition |
Most common causative fungi |
|
Superficial mycoses |
Fungal infections that involve
hair, skin or nails without direct invasion of the deeper tissue. |
Dermatophytes Candida Malassezia Piedraia |
|
Subcutaneous mycoses |
Fungal infections that are
confined to the subcutaneous tissues without dissemination to the distant
sites. |
Fonsecaea (Chromoblastomycosis) Sporothrix (Sporotrichosis) Madurella(Mycetoma) Cladosporium (Phaeohyphomycosis) |
|
Deep/invasive mycoses |
Fungal infections primarily
involving the lungs but may become widely disseminated & involve any
organ system. |
Histoplasma Blastomyces Coccidioides Paracoccidiodes |
|
Opportunistic mycoses |
Fungal infections that occur
primarily in patients who are immunocompromised. |
Aspergillus Zygomycetes (Mucor,Rhizopus) Candida Cryptococcus |
·
The superficial & subcutaneous
mycoses often produce characteristic lesions that strongly suggest their fungal
etiology. Sometimes it may resembles to some other clinical conditions.
·
In majority cases of systemic
mycoses, there are no signs or symptoms that specifically suggest a fungal
disease.
·
In such cases laboratory procedures
are used
o
to confirm the clinical suspicion
to establish fungal cause in disease of unknown etiology or
o To
exclude fungal involvement.
LABORATORY DIAGNOSIS
SPECIMEN COLLECTION:
The site as well as nature of specimens are most important to
proceed for final diagnosis.
a)
Superficial
mycoses:
Prior to collection of speciemen, clean the affected area
with 70% alcohol. (To remove any traces of skin products or medications)
Collect skin scales, crusts, pieces of nail or hairs in
sterile petri dish or paper envelope
·
Skin
scales:Dermatophytic lesions usually spread outward in
concentric fashion with healing in central region. Therefore, material should
be collected by scraping outward from edges of lesions with scalpel blade held
at an angle of 90O to skin surface.If multiple lesions are present,
then choose the most recent for scrapping as old loose scale is not
satisfactory.
When there is least scaling, as with lesions of glabrous
skin, it is preferable to use cellophane tape to take adequate material for
laboratory examination. (VAT preparation)
·
Hairs:
The specimen from the scalp are best obtained by scrapping
with blunt scalpel so that they include hair stubs, contents of plugged
follicles &scales.The base of the infected hairs should be collected by
clipping or plucking the hairs.
The
ringworm of scalp, especially caused by anthropophilic species may cause small
lesions that are difficult to detect clinically. This type of infection may
produce fluorescence of infected hairs& may be detected by wood’s lamp
examination which is helpful to detect scalp ringworm or to select material for
culture.
·
Nail
pieces: The nail clippings/scrapings should be taken from
discolored, dystrophicor brittle parts of nails. It should be collected from
proximal part of nails because fungus in distal part of nails is often
non-viable (fungus may be visible on
microscopy but fail to grow in culture)
b)
Subcutaneous
mycoses:
Scraping, crusts, aspirated pus & tissue biopsies are to
be collected aseptically from subcutaneous infections. Aspirated pus or biopsy
specimens are more valuable.
Mycetoma
When the mycetoma is caused by a fungus, the swelling is
called a Eumycetoma, and when caused by bacteria it is called an Actinomycetoma.
Actinomycetoma usually respond to treatment with antibiotics whereas Eumycetoma
are resistant to most antimicrobials. Therefore it is important to
differentiate between fungal & bacterial causes of mycetoma.
The color, size, consistency & microscopic appearance of
granules are used to diagnose & differentiate mycetoma. Black granules
indicate Eumycetoma, red granules anActinomycetoma& pale coloured granules
are produced in both type of mycetoma.
In mycetoma, the discharge from sinuses is aspirated after
cleaning the surface. Along with the discharge, granules must be collected.
When it is difficult to aspirate the pus, a sterile gauze piece is applied over
the sinus then pressureis applied over it to squeeze out some discharge. A
biopsy of the lesion may also be taken.
c)
Systemic/Invasive
mycoses:
In patients with invasive mycoses specimens may be taken from
as many as potential sites as possible. The sources include tissue biopsy, pus,
feces, urine, sputum, body fluids, blood, & scrapings & swabs from edge
of lesions.
II.
TRANSPORTATION
OF SPECIMENS:
Specimens are placed in sterile containers & transported
to the laboratory immediately. Specimens that are not processed immediately
should be held at room temperature.
The exception is ringworm specimens (skin, hairs, and nail
clippings), where the specimens can be placed in paper packages (rather than
screw cap containers) to reduce humidity & multiplication of bacteria.
Fungal elements can be detected in the clinical specimens by direct microscopic examination of material from the lesion.
v Potassium hydroxide (KOH) preparation
:
Keratinized tissue specimens such as skin scrapings and plucked hair samples are treated with 10% KOH which digests the keratin material so that the fungal hyphae will be clearly seen under the microscope. Heat the slide gently Over the flame and leave it aside for 5-10 minutes before examination.
· About 10% is the usual concentration of KOH used.
· About 20-40% KOH is needed for the specimens such as nail that otherwise takes longer time to dissolve.
· Biopsy specimens as they take longer time to dissolve, are usually dissolved in 10% KOH in a test tube and examined after overnight incubation Glycerol (10%) can be added to prevent drying DMSO (dimethyl sulfoxide) can be added to help in tissue digestion.
· Caution should be maintained while interpretation of hyphae, which may be confused with collagen fiber, cotton fiber or hair present in the clinical specimens.
v
Gram stain :
It is useful in identifying the yeasts (e.g. Cryptococcus) and yeast like fungi (e.g. Candida). They appear as gram-positive budding yeast cells.
v India ink and Nigrosin(Negative
) stains :
They are used as negative stains for demonstration of capsule of Cryptococcusneoformans.
v Calcofluor white stain:
It is more sensitive than other stains; binds to cellulose and chitin of fungal cell wall and fluoresce under UV light.
v Histopathological stains:
They are useful for demonstrating fungal elements from biopsy tissues. This is useful for detecting invasive fungal infection.
· Periodic acid schiff (PAS) stain : It is the recommended stain for detecting fungi. PAS positive fungi appear magenta/deep pink, whereas the nuclei stain blue.
· Gomorimethenamine silver (GMS) stain : It is used as an alternative to PAS for detecting fungi. It stains both live and dead fungi, as compared to PAS which stains only the live fungi. GMS stains the polysaccharide component of the cell wall. Fungi appear black whereas the background tissue takes pale green color.
· Mucicarminestain : It is used for staining the carminophilic cell wall of Cryptococcus and Rhinosporidium.
· Masson fontanastain : It is used for pigmented (or pheoid) fungi.
· Hematoxylin and Eosin (H and E) stain.
v
Lactophenol cotton blue (LPCB) :
It is used to study the microscopic appearance of the fungal isolates grown in culture. It contains:
· Phenol acts as disinfectant.
· Lactic acid preserves the morphology of fungi.
· Glycerol prevents drying.
· Cotton blue stains the fungal elements blue.
Observation- Fungi are stained blue and even the background is stained blue but fungal elements
like hyphae (septate,aseptate),conidia etc. can be well appreciated.
Culture
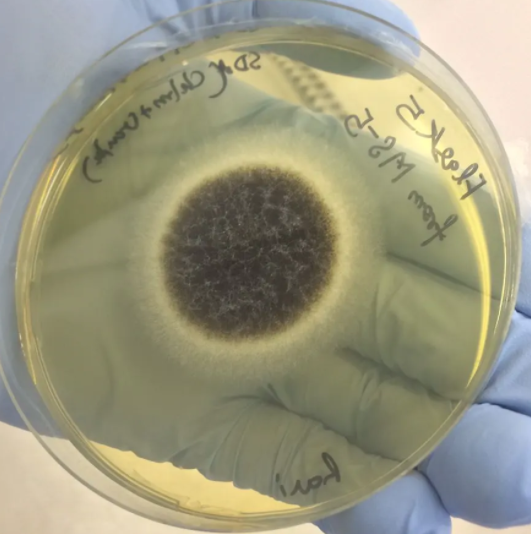
Fungal culture is frequently performed for isolation and correct identification of the fungi.
v Culture Media
· Sabouraud's dextrose agar (SDA) : It is the most commonly used medium in diagnostic mycology. It contains peptone (1%), dextrose (4%) and has a pH of 5.6. This may not support some pathogenic fungi.
· Neutral SDA (Emmons' modification) : It differs from original SDA in having neopeptone (1%) and dextrose (2%) and pH of 7.2.
· Corn meal agar and rice starch agar : They are the nutritionally deficient media used for stimulation of chlamydospore production.
· Brain heart infusion (BHI) agar and blood agar : They are the enriched media, used for growing fastidious fungi like Cryptococcus and Histoplasma.
· Niger seed agar and bird seed agar : They are used for the selective growth of Cryptococcus.
· CHROM agar Candidamedium : It is used as selective as well as differential medium for speciation of Candida.
· Czapek-Doxmedium :It is used as a selective growth medium,used for growing saprophytic fungi like Aspergillus, Candida, Penicillium and Paecilomyces.
v Culture Condition
· Temperature : Most of the fungi grow well at 25-30°C except the dimorphic fungi that grow at both 25°C and 37°C.
· BOD incubators (biological oxygen demand) : It is a special incubator used in diagnostic mycology, which is capable of maintaining low temperature.
· Incubation time : Culture plates should be incubated for 2-3 weeks. Cutures are routinely incubated in parallel at room temperature (22°C) for weeks and at 37°C for days.
· Antibiotics such as cycloheximide (actidione), chloramphenicol and gentamicin can be added to the culture media to inhibit bacterial growth.
v Culture Identification
The correct identification of the fungus is based on the macroscopic appearance of the colonies grown on culture and microscopic appearance (LPCB mount of colonies).
v Macroscopic Appearance of
the Colony
·
Rate of growth :
§ Rapid growth (<5 days) : It is seen in saprophytes, yeasts and agents of opportunistic mycoses.
§ Slow growth (1-4 weeks) : It is observed in dermatophytes, agents of subcutaneous and systemic mycoses.
· Colour and morphology of colony on the obverse
· Pigmentation : It can be seen on the reverse side of the culture media.
· Texture :It refers to how the colony would have felt if allowed to touch. It may be of various types such as — glabrous (waxy/leathery), velvety, yeast like, cottony or granular/powdery.
· Colony topography : Colony surface may be rugose (radial grooves), folded, verrucose or cerebriform (brain-like).
v Microscopic Appearance of
Fungi
· Teased mount : A bit of fungal colony Is teased out from the culture tube and the LPCB mount is made on a slide and viewed under microscope. If proper teasing is not done, then the intact morphology may not be identified properly. Identification is based on the following:
§ Nature of hyphae (such as septate or aseptate, hyaline or phaeoid, narrow or wide) and
§ Type of sporulation (conidia or sporangia).
· Slide culture : Though this is a tedious procedure, it gives the most accurate in situ microscopic appearance of the fungal colony. A sterile slide is placed on a bent glass rod in a sterile petri dish. Two square agar blocks measuring around 1 cm2 (smaller than the coverslip) are placed on the slide. Bits of fungal colony are inoculated onto the margins (at the center) of the agar block. Then the coverslip is placed on the agar block and the petri dish is incubated at 25°C. After sufficient growth occurs, LPCB mounts are made both from the coverslip and the underneath slide.
·
Cellophane tape mount : The impressions are taken by placing the cellophane tape on the
colonies present on the surface of SDA plate, then LPCB mount is made from the
cellophane tape. This is easy to perform than slide culture and in-situ
fungal morphology is also maintained.
v Other Methods of
Identification
· For Candida : Germ tube test, Dalmau plate culture, carbohydrate fermentation and carbohydrate assimilation tests are done.
· For dermatophytes : Hair perforation test, dermatophyte test medium and dermatophyte identification are used Urease test can be done for the fungi that produce urease medium enzyme, e.g. Cryptococcus.
Immunological Methods
These tests are available to detect the antibody or antigen from serum and/or other body fluids.
· Antibody detection can be done by ELISA, immunodiffusion test, agglutination test and complement fixation test (CFT).
· Antigen detection : Example includes latex agglutination test for detecting cryptococcal antigen from CSF, Candida and Aspergillus.
· Immunohistochemistry : It refers to detecting antigens (e.g. proteins) on the cells of a tissue section by using fluorescent tagged antibodies that bind specifically to the antigens. It is useful in deep mycoses.
Tests for Metabolites
An alternative approach for the diagnosis of fungal infections is detection of specific fungal metabolites in body fluids by gas liquid chromatography.
Tests to Demonstrate Delayed
Hypersensitivity
Skin tests are available for demonstrating delayed type of hypersensitivity for pathogens like Histoplasma, Blastomyces, Coccidioides, Paracoccidioides, Dermatophyte, Sporothrix and Candida.
Molecular Methods
Polymerase chain reaction (PCR) and its modifications such as multiplex PCR, nested PCR and the most advanced real time PCR and DNA sequencing methods have been developed for accurate identification of fungi from culture as well as from the specimens.

No comments:
Post a Comment