(Intestinal
parasites)
Preface:
Parasitic infestation: These are the parasitic diseases caused by the physical presence of the parasites on the surface of the host’s body. These may include those diseases which are caused by the presence of parasites in the intestines without invasion of the mucosa of the GI tract. These parasites are known as ecto-parasites.
Parasitic infection: These are the parasitic diseases caused by invasion of the deeper parts of the host’s body. These parasites are known as endo-parasites.
The intestinal parasites:These parasites reside in the gastro intestinal tract. They can complete their life cycle or wander in any part of the body; but most commonly involve intestines and produce intestinal manifestations.
Clinical Manifestations of parasitic infections of
intestine:
Intestinal manifestations:
Many parasitic diseases are associated with no or minimal GI symptoms. Abdominal discomfort is the most common presentation in any intestinal parasitic disease. This may or may not be associated with the following clinical presentations.
1. Dysentery: Entamoeba histolytica is the commonest to be associated with dysentry.
2. Steatorrhoea: Mainly associated with Giardia intestinalis.
3. Watery Diarrhoea: The parasites like Cryptosporidium parvum, Cyclospora sp. may cause watery diarrhoea.
4. Constipation: Majority of helminthic infestations are associated with abdominal discomfort with alternative diarrhoea and constipation.
5. Vomiting: It is a less common presentation and may be associated with any of the parasitic diseases.
Extra intestinal manifestations:
Many helminthic infestations are associated with malabsorption leading to anaemia and weight loss. Other symptoms may includes allergies, itching and pneumonia due to migrating larva.
Learning
objectives:
Collection of the specimens for detection of stool parasites:
Examination of stool specimen:
Gross examination:
Microscopic examination:
Techniques for concentration of parasites in the stool specimen
1. Sedimentation technique.
2. Floatation technique.
3. Techniques for concentration of motile larvae.
Microscopic methods
A. Wet mount preparation:
1. Saline preparation.
2. Iodine preparation.
B. Special staining methods:
1. Iron haematoxylin.
2. Modified Z N stain.
3. Trichrome stain.
Collection
of the specimens:
- Freshly collected stool specimen is always recommended for parasitic examination as it can also reveal motile forms of parasites, if they are present in the specimen.
- Stool should be collected in suitable, clean, wide mouth container.
- The specimen should not be contaminated with water, urine or disinfectants.
- Normally passed stool is recommended. However, samples obtained after purgatives or enema may be collected to recover parasites in certain conditions.
- Sample should be transported within one hour to the laboratory for observing motility of parasites. If delay in transport for more than 24 hours is suspected, it should be preserved by addition of formalin.
Examination
of stool specimen:
Gross
examination:
- On gross inspection of sample, adult worm may be seen in stool samples, e.g., round worm, thread worm, Fasciolopsis buski, segments of tape worms etc.
- Presence of mucus and blood will indicate dysentery, which is a common presentation of acute intestinal amoebiasis.
- Foul smelling white stool indicates steatorrhoea, which is a common presentation of giardiasis.
Microscopic
examination:
- Wet mount preparation of freshly passed sample will reveal motile forms of parasites i.e.
- Trophozoites of Entamoeba histolytica or Giardia intestinalis
- Larvae of Strongyloides stercoralis
- Wet mount preparation can easily reveal ova and cysts of parasites if they are abundant in the sample. If the sample contains fewer parasites, they may not be detected by direct microscopic examination. In such condition, they can be detected by using various concentration techniques prior to microscopic examination.
Techniques for concentration of parasites in the stool
specimen
1. Sedimentation techniques:
Formol-ether
sedimentation technique:
Procedure:
- 1 to 2 grams of stool is mixed with 10 to 15 ml of saline thoroughly.
- The mixture is filtered through a double layered gauze with the help of funnel. This will remove large particles.
- The filtrate is now centrifuged at 2000 rpm for 5 minutes.
- The supernatant is discarded and the sediment is mixed with 7 ml of formal saline. It is allowed to stand for 5 to 10 minutes.
- 3 ml of ether is added to the above suspension and vigorously mixed on vortex mixer.
- The suspension is now centrifuged at 2000 rpm for 2 minutes. The tube is now allowed to stand for a minute.
- Then, four distinct layers will be visible in the tube. The upper two layers are carefully discarded. The deposit at the bottom layer is resuspended in the 3rd layer of formal saline.
- A drop of suspension is placed on a slide and examined for the presence of parasitic ova / cysts.
2. Floatation techniques:
a.
Saturated salt solution floatation technique:
Procedure:
- 1 to 2 grams of stool sample is mixed with 3 - 4 ml of saturated salt solution in a test tube.
- After mixing thoroughly, more amount of saturated salt solution is added up to the brim of the test tube.
- A glass cover slip is placed on the brim of the above test tube, in such a way that the supernatant of the suspension remains in touch with the cover slip. It is allowed to stand like this for 30 minutes.
- After 30 minutes, the ova which tend to float in saturated salt solution will get concentrated in the supernatant at the air interface and hence, will get stuck to the cover slip.
- The cover slip is now picked up and placed on a clean glass slide for microscopic examination.
b.
Zinc sulphate floatation technique:
- 1 to 2 grams of stool is thoroughly mixed with 8 to 10 ml of distilled water.
- This suspension is filtered through a double layered gauze piece. The filtrates are again mixed with distilled water and centrifuged at 2000 rpm for 10 minutes
- The deposit is now mixed with saturated zinc sulphate solution and allowed to stand for 30 minutes.
- After 30 minutes, fluid from supernatant is carefully aspirated with a dropper and placed on a glass slide for microscopic examination.
3. Technique for concentration of motile larvae:
The motile larvae of S. stercoralis can be recovered from a solid stool sample by a simple method:
A small pit is created with a stick in the stool specimen. This pit is filled with warm saline. The motile larvae will migrate towards this saline. After 1 to 2 hours, saline from the pit is aspirated with a dropper and a drop of it is placed on the slide. This is covered with a cover glass and the preparation is examined under 10X objective. This can reveal a large number of motile larvae.
Microscopic
methods
A.
Wet mount preparation:
1.
Wet mount of dysenteric and unformed specimens:
A small amount of sample containing pus or mucus flakes is picked up with a wire loop or a stick and placed on a clean slide. It is covered with a cover glass. Using a tissue paper, press gently on the cover glass to make a thin preparation.
2.
Saline preparation:
This is used for the examination of semi formed and formed stool. A small amount of specimen is mixed with saline on a slide and covered with a cover glass.
3.
Iodine preparation:
Lugol's iodine (5%) or Dobeil's iodine (2%) may be used. A drop of iodine is mixed with a drop of stool suspension & examined under microscope. The iodine stains the nuclei and glycogen mass of cysts brownish black. This helps to identify the cysts.
4.
Eosin preparation:
A drop of eosin is mixed with a drop of stool suspension & examined under microscope. Eosin does not stain the trophozoites or cysts but provides a pink background against which the parasitic structure can easily be identified.
Wet
mount preparation should be thoroughly examined under 10X & 40X objectives
of microscope for following
structures:
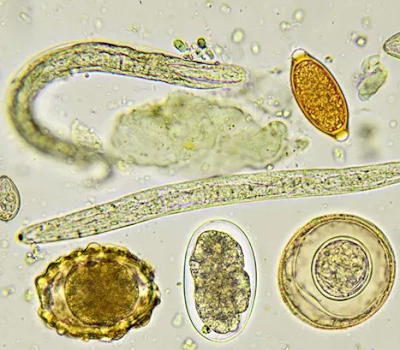
1. Larvae:
-S.stercoralis is the only larva that is found in stool.
- In freshly passed stool sample, motilility of the larvae can be appreciated in saline preparation.
- Non-motile larvae can also be easily identified under 10X objective due to their larger size.
2. Trophozoites:
- The direct wet mount preparation or saline preparation reveals motility of trophozoites.
- E.histolyticatrophozoites – They have very slow motility with the help of pseudopodia formation.
- G. intestinalistrophozoites - They have falling leaf like motility with four pairs of flagella.
- T. hoministrophozoites – They are actively motile. They can be easily identified due to their typical twitching motility.
Iodine preparation hampers the motility of trophozoites, as it kills the parasites, (iodine is a halogen) but it reveals their detailed internal structures like nuclei and glycogen mass.
3. Ova or eggs:
The size of helminthic ova is more than twice that of the protozoal cysts. They can be identified further by their specific morphological characteristics & size.
Ova of Cestodes:
- Size of the ova of Taenia: approximately 33-40 µm in diameter.
- Size of the ova of H. nana: approximately 40-50 µm in diameter.
- These ova show 3 pairs of hooklets.
Ova of Trematodes:
- Size of the ova of Clonorchis sinensis: 27-32 X 15-18 µm.
- Size of the ova of Fasciola hepatica: 130-145 X 70-90 µm.
- Size of the ova of Fasciolopsis buski: 130-145 X 70-90 µm.
- These ova show a distinct operculum.
Ova of Nematodes:
- The nematode ova are usually oval in shape & their size ranges approximately from 25 to 40 µm X 40 - 80 µm.
- Ova of Ascaris lumbricoides: these are bile stained & shell is covered by an uneven albuminous coat.
- Ova of Trichuris trichiura: these also are bile stained, but smaller than those of Ascaris& show mucus plugs on both the poles.
- Ova of Ancylostoma duodenale & Necator americanus: they are non-bile stained, have blastomeres or a larva within them. Commonly, 4 to 8 blastosmeres are seen within an ova.
- Ova of Enterobius vermicularis: they are non-bile stained, with one side plane & the other side convex, i.e., plano-convex.
4. Cysts:
- Cysts of E.histolytica measure about 10-15 µm in diameter, show 1, 2 or 4 nuclei, chromatid bars and sometimes glycogen mass also. These internal structures can be distinctively visualized in iodine preparation.
- Cysts of Giardia intestinalis measure about 10X6 µm. Internal structures include 4 nuclei grouped at one end, axoneme and median bodies. These structures can be more clearly & easily identified in iodine preparation.
5. Other structures:
- Presence of pus with or without blood suggests dysentery.
- Charcot - Leyden crystals may be present in stool sample; which are generally seen in parasitic infections & are particularly suggestive of amoebic dysentery.
B.
Special staining methods for fixed smear:
These staining methods help in identification of ova and cysts of the parasites. These are identified due to their distinct staining properties. Permanent slides can also be prepared & preserved for further reference and academic purposes.
1.
Iron haematoxylin:
This is used for differentiation among various amoebic cysts. This is done on the basis of specific & peculiar shapes of their nuclei.
2.
Modified Z N stain:
The oocysts of Cryptosporidium and Isospora are weakly acid fast. A smear is prepared from the stool suspension on a slide & is stained with hot or cold ZN staining method. Here, 1% H2SO4is used instead of 20% as the decolorizing agent.
3. Trichrome stain:
This staining method can be used for the demonstration of various protozoa. It is specially useful for the detection of very small oocysts of Microsporidia.
Table: The intestinal parasites found in stool specimen
|
Sr. No. |
Parasite |
Form of parasite found in stool |
|
Protozoa: |
||
|
1 |
Entamoebahistolytica |
Trophozoites,
cysts |
|
2 |
Giardia
intestinalis |
Trophozoites,
cysts |
|
Helminths
(worms): |
||
|
3 |
Taeniasolium,
Taeniasaginata (tape worms) |
Segments
of adult worm, ova(eggs) |
|
4 |
Hymenolepsis
nana(dwarf tape worm) |
Ova(eggs) |
|
5 |
Diphyllobothriumlatum |
Segments
of adult worm, ova(eggs) |
|
6 |
Schistosomajaponicum&Schistosomamansoni |
Ova
(eggs) |
|
7 |
Clonorchissinensis |
Ova
(eggs) |
|
8 |
Fasciola
hepatica |
Ova
(eggs) |
|
9 |
Fasciolopsisbuski |
Adult
worm, ova (eggs) |
|
10 |
Trichuristrichiura
(Whip worm) |
Ova
(eggs) |
|
11 |
Ancylostomaduodenale,
Necatoramericanus (Hook worm) |
Ova
(eggs) |
|
12 |
Ascarislumbricoides
(Round worm) |
Adult
worm, ova (eggs) |
|
13 |
Enterobiusvermicularis
(Thread worm / pin worm) |
Adult
worm, ova (eggs) |
|
Opportunistic
parasites causing infection in immuno-compromised patients: |
||
|
14 |
Cryptosporidium
parvum |
Oocysts |
|
15 |
Isospora
belli |
Oocysts |
|
16 |
Cyclosporacayetanensis |
Oocysts |
|
17 |
Microsporidia |
Oocysts |
|
18 |
Blastocystishominis |
Cysts |
|
19 |
Strongyloidesstercoralis |
Larva |
|
Non-pathogenic
parasites commonly found in diarrhoeal
stools: |
||
|
20 |
Trichomonashominis |
Trophozoites |
|
21 |
Entamoeba
coli |
Trophozoites,
cysts |
|
22 |
Balantidium
coli |
Trophozoites,
cysts |
Other
methods:
Duodenal contents
Examination of duodenal contents is useful for the detection of Giardia
lamblia and S. stercoralis, when repeated examination of faeces
fails to demonstrate these two parasites in the faeces. It is collected by
duodenal intubation.
Entero Test
It is a frequently used test to collect duodenal contents for microscopic examination and demonstration of the parasites. It uses a gelatin capsule attached to a thread containing a weight. One end of the thread is attached to the outer aspects of patient’s cheek , and then, the capsule is swallowed. Capsule gets dissolved in stomach releasing the thread which is carried to duodenum by peristalsis and due to weight attached to the thread. The thread gets unfolded and takes up duodenal samples. Four hours later, the thread is withdrawn and shaken in saline to release trophozoites which can be detected microscopically by wet mount or permanant stained smear.This procedure eliminates the need for duodenal intubation.
Anal Swabs
Cellophane tape
preparation
The principle of this method is that the eggs are deposited by female Enterobiusvermicularis on the surface of the skin surrounding the anus during night. These eggs stick to the sticking surface of cellophane tape, which can be visualised under the microscope.
The slide is examined microscopically under a low-power objective
(10×) of the microscope with low
illumination. The specimen is best collected in the morning before bathing and
going to the toilet. The cellophane tape method occasionally detects the eggs
of Taenia spp. and Schistosomamansoni.

No comments:
Post a Comment